|
Rescooped by Lionel Reichardt / le Pharmageek from Digital Pharma news |
Get Started for FREE
Sign up with Facebook Sign up with X
I don't have a Facebook or a X account
 Your new post is loading... Your new post is loading...
 Your new post is loading... Your new post is loading...
No comment yet.
Sign up to comment

rob halkes's curator insight,
August 1, 2016 4:50 AM
Health apps should do what they promise! At the moment they need to take a diagnostic feature and use personal physics to arrive at advice or conclusions about the health status of the person who uses the app, they are considered not to be 'just' an "app" but a medical device. At that condition they need to adhere to and be certified by several criteria attached to 'medical devices". Developers should know about this, which the more professional ones will. Rightly so! PatientView has developed a website MyHealthApps that presents an inventory of the better Health Apps. 
Pharma Guy's curator insight,
August 1, 2016 8:38 AM
Also read “FDA Won't Regulate ‘Low-Risk’ mHealth Apps as Medical Devices. But Battle Looms Over Defining ‘Low Risk’"; http://sco.lt/5kkDyr

Seth Bilazarian, MD's curator insight,
April 24, 2013 4:25 PM
"There's an app for that." Physicians are often criticized for not doing a better job reporting adverse events and this is largely because the method for reporting to the FDA has been burdensome and difficult. An easy to use reporting strategy from a smartphone will increase my reporting dramatically. The speed of reporting and analysis by FDA for actionable items should be significantly shortened. 
Seth Bilazarian, MD's comment,
April 24, 2013 4:27 PM
#app, medical app, #chealth, #mHealth, FDA, adverse event reporting, Bilazarian
|

Pharma Guy's curator insight,
September 15, 2017 7:56 AM
When I first saw this story, I thought this app also helped patients who are addicted to opioids. I wonder if anything could solve that problem! 
Richard Platt's curator insight,
September 29, 2017 10:40 PM
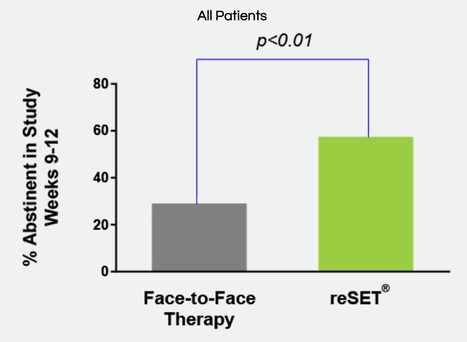
The FDA’s approval came on the back of a 12-week clinical trial involving 399 patients on either standard treatment or standard treatment plus a desktop version of Reset. It showed a statistically significant increase (40.3% vs 17.6%) in adherence to abstinence for patients with alcohol, cocaine, marijuana and stimulant SUD who used Reset. The trial did not demonstrate the effectiveness of using Reset for opioid abuse and the application is not licensed to treat opioid dependence. Carlos Peña, director of the Division of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health, said: “This is an example of how innovative digital technologies can help provide patients access to additional tools during their treatment. “More therapy tools means a greater potential to help improve outcomes, including abstinence, for patients with substance use disorder.” Reset contains a patient application and clinician dashboard and is indicated as a prescription-only adjunct treatment for patients with SUD who are not currently on opioid replacement therapy, do not abuse alcohol solely or whose primary substance of abuse is not opioids. However, Pear’s development pipeline does include a version of the app specifically for opioid use disorder, alongside devices for use in areas such as schizophrenia, pain and major depressive disorder.

Pharma Guy's curator insight,
April 16, 2016 6:59 AM
Related: "An Analysis of Genentech's 4HER Mobile Health App Privacy Policy"; http://bit.ly/4HERapp and "mHealth App Developers Ask for HIPAA Clarity"; http://bit.ly/mAppHIPAA I used this tool thinking of a few pharma mHealth apps I have seen. To see the results of that exercise, click here: http://bit.ly/mHealthAppQandA

rob halkes's curator insight,
January 16, 2014 5:33 AM
Well, I agree very much with David about the potential and the benefits of using mobile technology in trial studies. Yet, I think that the very patients are entitled to not just that. I know of cases in which patients have been supported and guided on an intensive schedule to their participation in the trial. But, alas, when the trial was over, so the support and guidance disappeared. Why not continue this in the very support at therapy? My take is that both FDA and EMA should take steps to guarantee that services rendered during trials should be continued, by the participating institutes and business of care and research to the services for the participating patients. Isn't that self evident? Please endorse this, by your comments!
|